BY: LIZAWATI MADFA
SHAH ALAM, JAN 6: Hypertension patients, treated with Ternolol 50 Film-Coated Tablet manufactured by Hovid Berhad, are advised to check whether they have the drug with batch number BG04645.
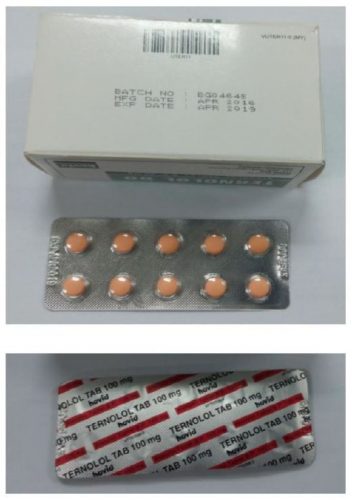
Health Ministry Director Dato’ Dr Noor Hisham Abdullah said National Pharmaceutical Regulations Unit (NPRA) has issued instructions to the manufacturer to withdraw that particular batch.
He said the instruction was issued due to the label on the external box says Ternolol 50 but the label on the product blister is printed as Ternolol Tab 100 milligrams (100 mg).
“If any patients has that batch in their stock, we seek their cooperation to not consume the medication and return it immediately to any clinic, pharmacy or health facility from which they had gotten the supply.
“Please see a medical practitioner for further advice or examination if necessary,” he said in a statement.
He said NPRA and the manufacturer are conducting a comprehensive investigation to identify the cause of the issue.
“The product is registered by the Drug Control Authorities (PBKD) with the registration number MAL19940057AZ.
“Ternolol 50 Film-Coated Tablet contains atenolol as the active ingredient prescribed for hypertension treatment,” he said.
The public can contact NPRA at 03-78835538 or the National Pharmacy Call Centre (NPCC) at 1800 88 6722.
Health Ministry withdraws hypertension drug





