PUTRAJAYA, March 19 — The Health Ministry (MoH) has revoked the notification of three cosmetics products as they were found to contain scheduled poisons and are prohibited from being sold in Malaysia.
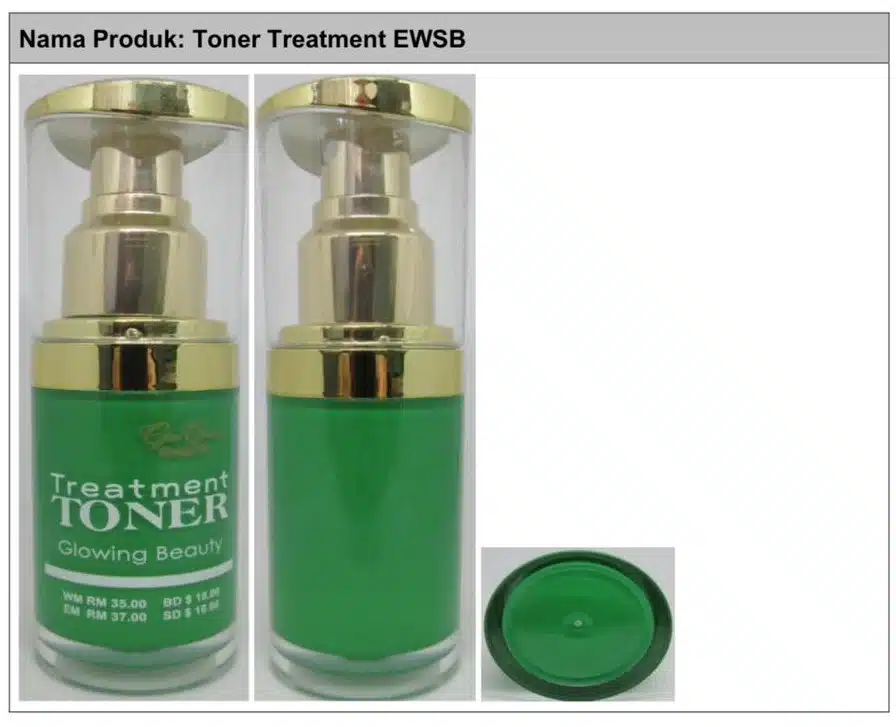
Its director-general Datuk Dr Muhammad Radzi Abu Hassan said the products include Toner Treatment EWSB, which contains hydroquinone and tretinoin; N Glow EWSB (hydroquinone, tretinoin, and betamethasone 17-Valerate), and Glory Cosmetics Facecream (mercury).
The notifications of these three products were revoked by the ministry’s senior director of pharmaceutical services, and these items are not allowed to be sold in the country.
“Mercury is prohibited in cosmetic products as it can be absorbed into the body and damage the kidneys and nervous system.
“It can also interfere with the brain development of young children or foetuses. Mercury can also cause rashes, irritation, and other changes to the skin,” he said in a statement today.
Dr Radzi said these products containing hydroquinone, tretinoin, and betamethasone 17-Valerate are drugs that must be registered with the Drug Control Authority and can only be used with the advice of health professionals.

The use of products containing these ingredients without the supervision of health professionals can cause unwanted side effects.
When hydroquinone is applied to the skin, it can cause redness, while tretinoin can cause redness, discomfort, soreness, peeling, and hypersensitivity to sunlight, among others.
Meanwhile, betamethasone 17-Valerate can cause part of the facial skin to become thin and prone to irritation, acne, and skin pigmentation. It can also be absorbed into the blood circulation system, which can have harmful effects.
Accordingly, he urged the sellers and distributors of these products involved to immediately stop the sale and distribution of all the products in question for violating the Drugs and Cosmetics Control Regulations 1984.
Individuals who commit offences under the regulation face a maximum penalty of a fine of RM25,000 or imprisonment of three years or both for the first offence and a fine of RM50,000 or imprisonment of five years or both for subsequent offences.
Companies that commit offences face a maximum fine of RM50,000 for the first offence and RM100,000 for subsequent offences.
“The public who are currently using these cosmetic products are advised to immediately stop using them and seek advice from health professionals if they experience any discomfort or adverse effects,” Dr Radzi said.
— Bernama