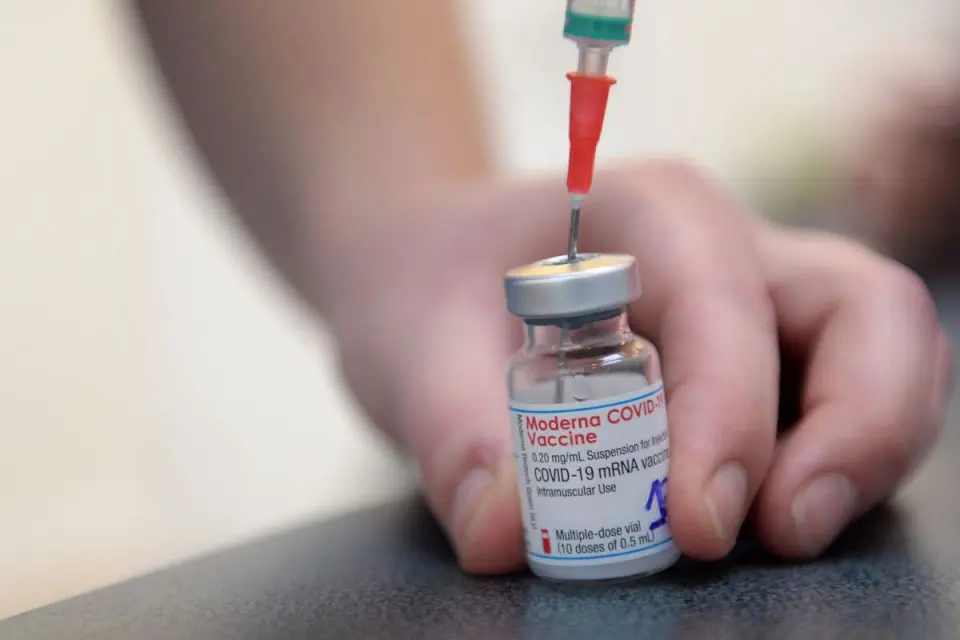
KUALA LUMPUR, Aug 5 — The Drug Control Authority (DCA) in its 362nd meeting today, gave conditional registration approval to Spikevax 0.20 mg/ml Dispersion for Injection Covid-19 mRNA Vaccine (nucleoside modified), also known as Covid-19 Moderna vaccine for use in the current disaster situation.
Health director-general Tan Sri Dr Noor Hisham Abdullah in his statement today said approval for the product was for product registration holder (PRH), Zuellig Pharma Sdn Bhd and the manufacturer, Rovi Pharma Industrial Services of Spain.
“The conditional registration approval required quality information as well as the efficacy of the vaccine to be monitored and evaluated based on the latest data from time to time.
“It is to ensure the comparison of benefit over risk for the vaccine product remains positive,” he said.
Dr Noor Hisham said the Health Ministry is committed to improve access to the Covid-19 vaccines in Malaysia by ensuring the products are evaluated in terms of quality, safety and effiicacy aspects by the National Pharmaceutical Regulatory Agency (NPRA) and approved by DCA.
— Bernama